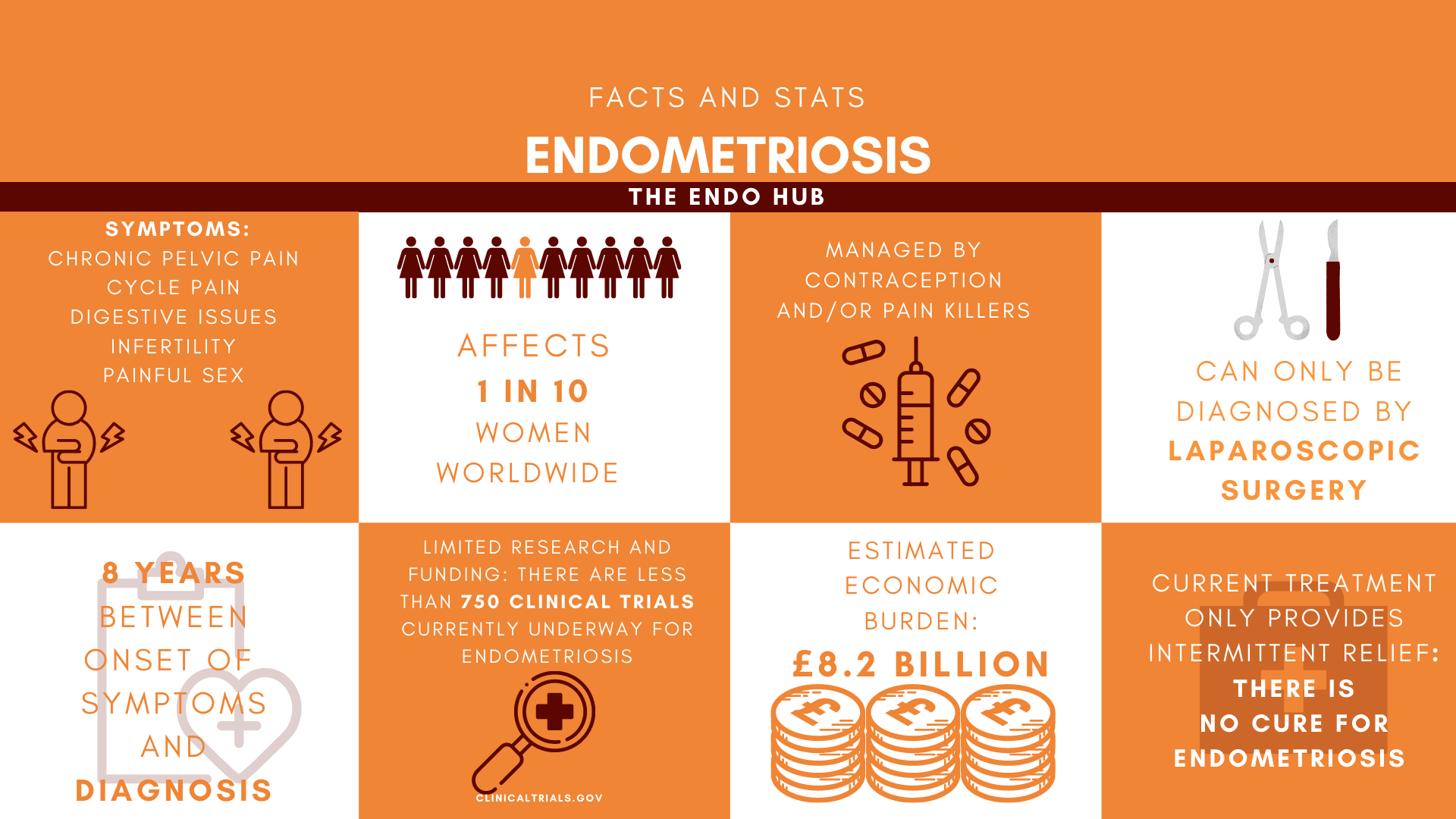
Endometriosis remains mysterious to those living with it, as well as those treating it. As you can imagine, it’s hard to treat an illness when you’re not sure what exactly is causing it. Trying to manage my own endo without knowing what’s really going on in my body has felt like I’m just swinging with my eyes closed, hoping to hit my opponent by chance. This in part explains why treatment for endometriosis can be so hit and miss, but what are some of the possible causes for endometriosis?
What causes endometriosis?
Currently, there is no one definable cause for endometriosis (annoying, I know). However, there are many different theories to explain what could cause endometriosis. This article will touch on a few of the popular theories behind the disease.
Retrograde Menstruation
Retrograde menstruation is thought to cause endometriosis due to the ‘back flow’ of menstrual blood into the fallopian tubes and the within the abdomen. This ‘back flow’ is thought to carry endometrial tissue that sticks to pelvic surfaces, creates a blood supply, and grows with each menstrual cycle. As these cells grow with each cycle and create lesions, they cause inflammation and pain, as they are unable to leave the body the normal way menstrual blood usually does.
Although retrograde menstruation explains how endometrial cells ‘get lost’, it does not explain how they are able to stay in that location and develop into lesions. If this theory alone causes endometriosis, it would occur in the 90% of the menstruating population who also experience retrograde menstruation and wouldn’t occur in those who don’t menstruate. Retrograde menstruation also implies that this disease worsens with age (which it does not), as the endometrial lesions would increase in size in every cycle. A number of processes are needed to sustain the lesions and keep them alive (we all need food, right?). These mechanisms include avoiding immune system clearance, attaching to pelvic cell surfaces, and establishing new blood and nerve supply. Whether these processes follow through or not depends on your genes, the oestrogen-progesterone balance, and inflammation. Although this theory may explain how endometriosis can be triggered, it is not the sole cause.
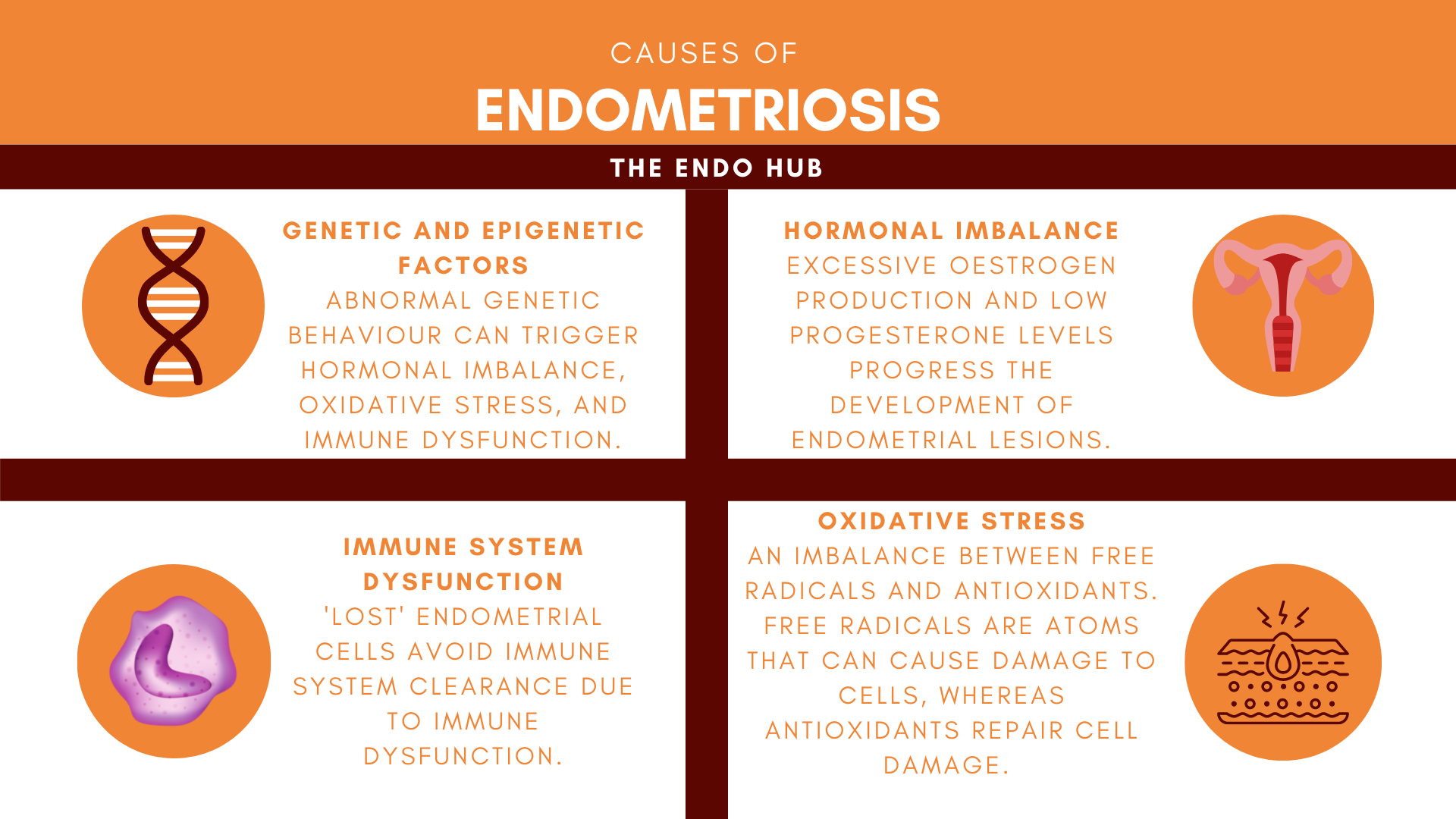
Hormonal Imbalance
An imbalance between sex hormones oestrogen and progesterone can increase the ability of endometrial lesions to develop (i.e attach to pelvic surfaces, establish a blood supply and evade immune clearance). Oestrogen and progesterone work tightly together to coordinate a regular menstrual cycle by sending messages to organs in the body, through receptors. Receptors are cells on the surfaces of organs that recognise molecules and allow their messages to be delivered (think of the hormones as a letter, and the receptor as a postbox). The two hormones directly influence one another: oestrogen mainly functions to aid in the development of breasts, pubic hair, etc., and regulates the menstrual cycle. Progesterone helps to prepare the body for pregnancy and also regulates the number and sensitivity of oestrogen receptors. The levels of the two hormones must be balanced in order to correctly instruct cells on how to behave, however, in endometriosis, the balance between these two tightly kindled hormones is disrupted.
The two main receptors for oestrogen are ERα (oestrogen receptor alpha) and ERβ (oestrogen receptor beta). Oestrogen comes in three forms: estrone, estradiol, and estriol. In the normal endometrium, estradiol binds to ERα receptors, however, in endometriosis, endometrial lesions are able to create more estradiol and also contain higher amounts of ERβ receptors. This means the oestrogen receptors take a double hit, and must accept both circulating estradiol as well as the newly produced estradiol, from the lesions. More ERβ receptors results in more estradiol production, causing further growth of endometrial lesions.
The two main progesterone receptors are progesterone receptor A (PGR-A) and progesterone receptor B (PGR-B). PGR-A has typically been shown to ‘pump the brakes’ on estradiol induced growth, and also convert it to a less potent form of oestrogen. In endometriosis, studies have shown levels of PGR-A to be extremely low, and low PGR-A means less or no pumping the brakes on estradiol levels, promoting its creation.
Low expression of PGR-A receptors and higher levels of ERβ receptors ends in chaos: an imbalance in oestrogen and progesterone in endometriosis patients. This hormonal imbalance is able to help endometrial lesions to build and maintain a blood and nerve supply, as oestrogen is a key regulator of this process (neuroangiogenesis).
Oxidative Stress and Inflammation
Oxidative stress occurs when there is an imbalance between reactive free radicals and antioxidants. Increased oxidative stress has been linked to the ability of endometrial cells to divide outside of the endometrium. Free radicals are produced to regulate cell division, whereas antioxidants limit free radical production and repair cell damage. Oxidative stress is thought to further progress endometriosis, as an increase of free radicals has been shown to increase the growth of endometrial lesions. As free radicals are responsible for cell division, without enough antioxidants to ‘keep them in check’, they can cause these lost endometrial cells to divide more than usual.
Immune System Dysfunction
A key feature of endometriosis includes ‘lost’ endometrial cells escaping immune system clearance from the space between the abdomen and pelvis (peritoneum). The main cells involved in this immune system clearance are natural killer cells (NK Cells) and macrophages. NK cells act as bodyguards and are meant to escort ‘lost’ endometrial cells out of the peritoneum by breaking them down and clearing them out. In endometriosis, studies have shown ‘lost’ endometrial cells to be more resistant to breakdown by NK cells. Picture the NK Cells as bodyguards and the peritoneum as a highly secured building that only those with security clearance can enter. In endometriosis, the ‘lost’ cells are able to bypass the bodyguards by using fake IDs.
Genetic and Epigenetic Factors
Endometriosis has been found to have a 51% heritability rate, and be more common amongst first, second, and third-degree relatives such as aunts, cousins, and mothers and sisters. Genetics is the study of how genes about our health, physical appearance, and personality are passed on from parents to offspring. Genetics often explains why people with the same disease or health issue may not share the same experience. Your DNA holds your unique genetic code, which acts as an instruction manual on which cells to create in the body. Gene expression describes the process by which the instructions in our DNA are turned into a ‘functional’ product. While genetics decides which cells are made, epigenetics decides how genes behave, acting as an ‘on/off’ switch that sits on top of the DNA. In disease, however, abnormal patterns in gene expression occur in response to our environments, leading to poor functioning of tissues and organs.
All the factors mentioned above such as hormonal imbalance, oxidative stress, and immune system dysfunction can be affected by epigenetic factors. Through epigenetics, genes can absorb environmental factors and directly influence how cells behave. Abnormal epigenetic processes have been linked to ‘harmful’ cell function such as growth, development of new blood vessels, and cell migration.
There are genes that are responsible for all the cells and molecules in your body (genetic encoding). Remember earlier when we talked about hormonal imbalance and the receptors for oestrogen and progesterone? Well, those receptors also have genes that are responsible for how those receptors function: oestrogen receptor 1 (ESR1) encodes for ERα, and oestrogen receptor 2 (ESR2) encodes for ERβ. Studies have found increased levels of ESR2 and decreased levels of ESR1 in endometrial lesions of endometriosis patients. Abnormal gene expression for the genes responsible for uterine development has been seen in endometriosis. These changes in gene expression then have a knock-on effect on a variety of genes involved in uterine function, such as increased levels of ESR2 which encodes for oestrogen receptors.
That’s a lot… So what now?
Hopefully, this article has shed some light on the mystery behind endometriosis. We have come a long way from Samson’s Theory of retrograde menstruation as being the sole cause- but still have a long way to go. It’s becoming more clear that endometriosis is not a disease with one cause, but many, which would explain the differences of symptoms in those with the disease. With that being said, further investigation of all the above factors mentioned in this article would help to find out whether they are a cause of a consequence of endometriosis.
How did you find this article? Leave a comment down below! Hope you’re all managing well- remember, you’re not alone.
Joelle
References
Burney, R. and Giudice, L., 2012. Pathogenesis and pathophysiology of endometriosis. Fertility and Sterility, 98(3), pp.511-519.
Chantalat, E., Valera, M., Vaysse, C., Noirrit, E., Rusidze, M., Weyl, A., Vergriete, K., Buscail, E., Lluel, P., Fontaine, C., Arnal, J. and Lenfant, F., 2020. Estrogen Receptors and Endometriosis. International Journal of Molecular Sciences, 21(8), p.2815.
Carvalho, L., Samadder, A., Agarwal, A., Fernandes, L. and Abrão, M., 2012. Oxidative stress biomarkers in patients with endometriosis: systematic review. Archives of Gynecology and Obstetrics, 286(4), pp.1033-1040.
Creative-diagnostics.com. 2021. Estrogen and Progesterone. [online] Available at: https://www.creative-diagnostics.com/blog/index.php/estrogen-and-progesterone/
[Accessed 10 July 2021].
Dyson, M., Roqueiro, D., Monsivais, D., Ercan, C., Pavone, M., Brooks, D., Kakinuma, T., Ono, M., Jafari, N., Dai, Y. and Bulun, S., 2014. Genome-Wide DNA Methylation Analysis Predicts an Epigenetic Switch for GATA Factor Expression in Endometriosis. PLoS Genetics, 10(3), p.e1004158.
Han, S., Jung, S., Wu, S., Hawkins, S., Park, M., Kyo, S., Qin, J., Lydon, J., Tsai, S., Tsai, M., DeMayo, F. and O’Malley, B., 2015. Estrogen Receptor β Modulates Apoptosis Complexes and the Inflammasome to Drive the Pathogenesis of Endometriosis. Cell, 163(4), pp.960-974.
Healthline. 2021. Is Endometriosis Hereditary? The Genetics of Endometriosis. [online] Available at:
https://www.healthline.com/health/endometriosis/is-endometriosis-hereditary#treatment
[Accessed 10 July 2021].
Healthline. 2021. Oxidative Stress: Definition, Effects on the Body, and Prevention. [online] Available at: https://www.healthline.com/health/oxidative-stress#effects
[Accessed 9 July 2021].
KOUKOURA, O., SIFAKIS, S. and SPANDIDOS, D., 2021. Deregulation of gene expression in disease | Gene expression and chromatin signalling | The University of Manchester. [online] Bmh.manchester.ac.uk. Available at: https://www.bmh.manchester.ac.uk/research/domains/cellular-development-systems/gecs/deregulation-of-gene-expression/
[Accessed 10 July 2021].
Kumar, P., Amreen, S., Gupta, P. and Rao, P., 2019. Evaluation of oxidative stress and severity of endometriosis. Journal of Human Reproductive Sciences, 12(1), p.40.
Macer, M. and Taylor, H., 2012. Endometriosis and Infertility. Obstetrics and Gynecology Clinics of North America, 39(4), pp.535-549.
Neidler, S., 2021. Endometriosis and Retrograde Menstruation. [online] Endometriosis News. Available at: https://endometriosisnews.com/endometriosis-and-retrograde-menstruation/
[Accessed 10 July 2021].
Rolla, E., 2019. Endometriosis: advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Research, 8, p.529.
Scutiero, G., Iannone, P., Bernardi, G., Bonaccorsi, G., Spadaro, S., Volta, C., Greco, P. and Nappi, L., 2017. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxidative Medicine and Cellular Longevity, 2017, pp.1-7.
Seckin Endometriosis Center, 2021. Causes of Endometriosis: Several Proposed Theories. [online] Seckin Endometriosis Center. Available at: https://drseckin.com/retrograde-menstruation-cause-of-endometriosis/
[Accessed 10 July 2021].
Wang, Y., Nicholes, K. and Shih, I., 2020. The Origin and Pathogenesis of Endometriosis. Annual Review of Pathology: Mechanisms of Disease, 15(1), pp.71-95.
Yilmaz, B. and Bulun, S., 2019. Endometriosis and nuclear receptors. Human Reproduction Update, 25(4), pp.473-485.